Antioxidant and chemotherapeutic synergy: Triphala enhances doxorubicin cytotoxicity in breast cancer cells and reduces toxicity in non-tumorigenic cells
Keywords:
antioxidant therapy, breast cancer, chemotherapy, doxorubicin, gallic acid, reactive oxygen species, synergistic effect, triphalaAbstract
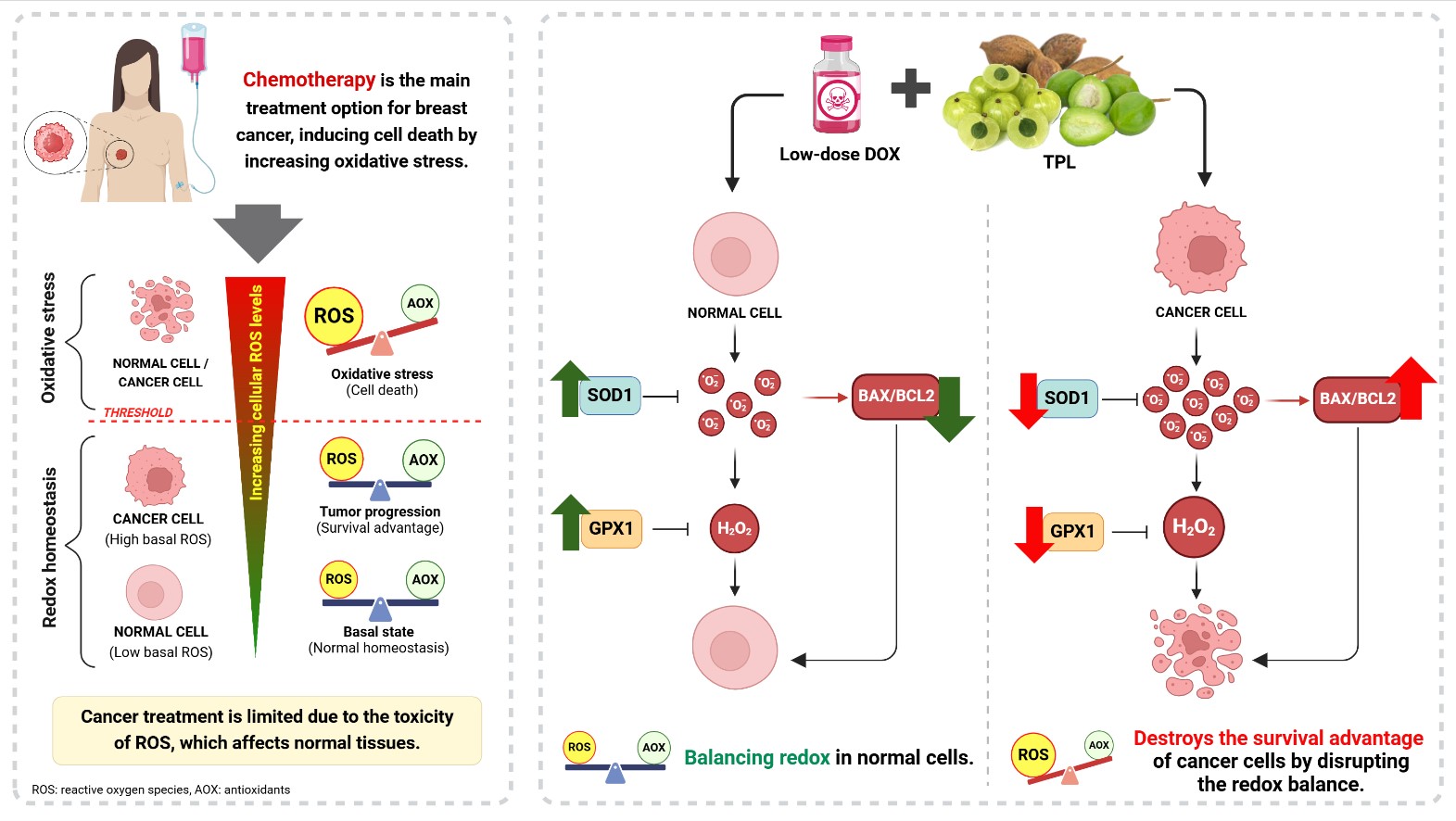
Background: The combination of antioxidants with chemotherapy is increasingly being explored to minimize toxicity in noncancerous cells during breast cancer treatment. Triphala (TPL), a Thai herbal compound rich in antioxidants, shows potential as a complementary candidate for use in breast cancer chemotherapy.
Objective: This study investigated the combined effects of TPL and low-dose doxorubicin (DOX) on human breast cancer and non-tumorigenic mammary epithelial cells.
Methods: TPL’s bioactive compounds were analyzed using high-performance liquid chromatography. Cell viability and the levels of reactive oxygen species (ROS) were assessed in breast cancer (MDA-MB-231, MCF-7) and epithelial (MCF-10A) cells using MTT and chloromethyl 22 ,72- dichlorodihydrofluorescein diacetate assays performed in triplicate. The combination index (CI) values were determined by CompuSyn. Furthermore, the mRNA expression of apoptosis- and antioxidant-related genes was evaluated using qPCR.
Results: Gallic acid (11.9%) was identified as the major component in TPL. The combination of TPL and lowdose DOX synergistically enhanced the cytotoxicity in MCF-7 and MDA-MB-231 cells. This combination significantly reduced the expression of the antioxidant genes SOD1 and GPX1 in MDA-MB-231 (SOD1: P < 0.001, GPX1: P = 0.035) and MCF-7 (SOD1: P = 0.035, GPX1: P = 0.036) cells, which resulted in increased ROS levels in MDA-MB-231 (P = 0.005) and MCF-7 (P = 0.008) cells. Elevated ROS triggered apoptosis via the increased BAX/BCL2 ratio in MDA-MB-231 (P < 0.001) and MCF-7 (P = 0.02) cells. Conversely, TPL displayed protective effects in nontumorigenic MCF-10A cells by upregulating SOD1 (P = 0.031) and GPX1 (P < 0.001), reducing ROS (P = 0.002), and lowering the BAX/BCL2 ratio (P < 0.001), thereby promoting cell survival.
Conclusion: TPL, in combination with low-dose DOX, effectively induces cytotoxicity in breast cancer cells while protecting non-tumorigenic cells, which suggests its potential as complementary therapy in breast cancer treatment.
Downloads
References
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21:363-83.
https://doi.org/10.1038/s41580-020-0230-3
Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008;4:89-96.
https://doi.org/10.59566/IJBS.2008.4089
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010;107:8788-93.
https://doi.org/10.1073/pnas.1003428107
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013;12:931-47.
https://doi.org/10.1038/nrd4002
Giaquinto AN, Sung H, Newman LA, Freedman RA, Smith RA, Star J, et al. Breast cancer statistics 2024. CA Cancer J Clin 2024;74:477-95.
https://doi.org/10.3322/caac.21863
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52.
https://doi.org/10.1038/35021093
Sarmiento-Salinas FL, Delgado-Magallón A, MontesAlvarado JB, Ramírez-Ramírez D, Flores-Alonso JC, Cortés-Hernández P, et al. Breast cancer subtypes present a differential production of reactive oxygen species (ROS) and susceptibility to antioxidant treatment. Frontiers Oncology 2019;9:480.
https://doi.org/10.3389/fonc.2019.00480
Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, et al. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res 2018;37:266.
https://doi.org/10.1186/s13046-018-0909-x
Meredith AM, Dass CR. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J Pharm Pharmacol 2016;68:729-41.
https://doi.org/10.1111/jphp.12539
Wargo JA, Reuben A, Cooper ZA, Oh KS, Sullivan RJ. Immune effects of chemotherapy, radiation, and targeted therapy and opportunities for combination with immunotherapy. Semin Oncol 2015;42:601-16.
https://doi.org/10.1053/j.seminoncol.2015.05.007
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol 2018;80:50-64.
https://doi.org/10.1016/j.semcdb.2017.05.023
Hou JG, Jeon BM, Yun YJ, Cui CH, Kim SC. Ginsenoside Rh2 Ameliorates Doxorubicin-induced senescence bystander effect in breast carcinoma Cell MDA-MB- 231 and normal Epithelial Cell MCF-10A. Int J Mol Sci 2019;20:1244.
https://doi.org/10.3390/ijms20051244
Zhang T, He L, Sun W, Qin Y, Zhang P, Zhang H. 1,25Dihydroxyvitamin D3 enhances the susceptibility of anaplastic thyroid cancer cells to adriamycininduced apoptosis by increasing the generation of reactive oxygen species. Mol Med Rep 2019;20:2641-8.
https://doi.org/10.3892/mmr.2019.10530
Simone CB 2nd, Simone NL, Simone V, Simone CB. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 2. Altern Ther Health Med 2007;13:40-7.
Wang W, Ige OO, Ding Y, He M, Long P, Wang S, et al. Insights into the potential benefits of triphala polyphenols toward the promotion of resilience against stress-induced depression and cognitive impairment. Curr Res Food Sci 2023;6:100527.
https://doi.org/10.1016/j.crfs.2023.100527
Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mishra KP, Banavalikar MM, et al. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res 2005;19:582-6.
https://doi.org/10.1002/ptr.1515
Sandhya T, Mishra KP. Cytotoxic response of breast cancer cell lines, MCF 7 and T 47 D to triphala and its modification by antioxidants. Cancer Letters 2006;238:304-13.
https://doi.org/10.1016/j.canlet.2005.07.013
Murthy MS. Anticancer and pro-apoptotic effects of Triphala extract in human breast cancer MCF-7 cells. Faseb J 2018;32:151.2.
https://doi.org/10.1096/fasebj.2018.32.1_supplement.151.2
Jagetia GC, Malagi KJ, Baliga MS, Venkatesh P, Veruva RR. Triphala, an ayurvedic Rasayana drug, protects mice against radiation-induced lethality by free-radical scavenging. J Altern Complement Med 2004;10:971-8.
https://doi.org/10.1089/acm.2004.10.971
Khan HY, Zubair H, Faisal M, Ullah MF, Farhan M, Sarkar FH, et al. Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: a mechanism for cancer chemopreventive action. Mol Nutr Food Res 2014;58:437-46.
https://doi.org/10.1002/mnfr.201300417
Moghtaderi H, Sepehri H, Delphi L, Attari F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. Bioimpacts 2018;8:185-94.
https://doi.org/10.15171/bi.2018.21
Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, et al. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biol 2015;6:461-71.
https://doi.org/10.1016/j.redox.2015.08.021
Jantarach PC, Chaiyawatthanananthn P, Thongdeeying P, Itharat A. Quality control standard values and stability study of ethanolic Ya-Hom KAELOM-WING-WIEN remedy extract on nitric oxide inhibition in LPS-stimulated RAW 264.7 macrophage cells of its anti-inflammatory activity. Thammasat Med J 2018;18:510-8.
Banlangsawan N, Sripanidkulchai B, Sanoamuang N. Investigation of antioxidative, antityrosinase and cytotoxic effects of extract of irradiated oyster mushroom. Songklanakarin J Sci Technol 2016; 38:31-9.
Sangweni NF, Dludla PV, Chellan N, Mabasa L, Sharma JR, Johnson R. The implication of low dose dimethyl sulfoxide on mitochondrial function and oxidative damage in cultured cardiac and cancer cells. Molecules 2021;26:7305.
https://doi.org/10.3390/molecules26237305
Zhu J, Yi X, Zhang J, Chen S, Wu Y. Chemical profiling and antioxidant evaluation of Yangxinshi Tablet by HPLC-ESI-Q-TOF-MS/MS combined with DPPH assay. J Chromatogr B Analyt Technol Biomed Life Sci 2017;1060:262-71.
https://doi.org/10.1016/j.jchromb.2017.06.022
Meena AK, Narasimhaji CV, Velvizhi D, Singh A, Rekha P, Kumar V, et al. Determination of gallic acid in ayurvedic polyherbal formulation triphala churna and its ingredients by HPLC and HPTLC. Res J Pharmacy Technol 2018;11:3243-9.
https://doi.org/10.5958/0974-360X.2018.00596.6
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 2006;58:621-81.
https://doi.org/10.1124/pr.58.3.10
Chou TC, Talalay P. Quantitative analysis of doseeffect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 1984;22:27-55.
https://doi.org/10.1016/0065-2571(84)90007-4
Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 2009;14:125-32.
https://doi.org/10.1016/j.tplants.2008.12.003
Pompimon W, Wattananon S, Udomputtimekakul P, Baison W, Sombutsiri P, Chuajedton A, et al. HPLC determination of the gallic acid and chebulinic acid contents of phyllanthus emblica linn., terminalia bellirica roxb., terminalia chebula retz. and triphala products from Chae Son district, Lampang, Thailand. Am J Food Sci Technol 2020;8:87-98.
Ran F, Han X, Deng X, Wu Z, Huang H, Qiu M, et al. High or low temperature extraction, which is more conducive to Triphala against chronic pharyngitis? Biomed Pharmacother 2021;140:111787.
https://doi.org/10.1016/j.biopha.2021.111787
Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 2016;7:e2253.
https://doi.org/10.1038/cddis.2016.105
Zhu H, Sarkar S, Scott L, Danelisen I, Trush MA, Jia Z, et al. Doxorubicin redox biology: redox cycling, topoisomerase inhibition, and oxidative stress. React Oxyg Species (Apex) 2016;1:189-98.
https://doi.org/10.20455/ros.2016.835
Nakamura H, Takada K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci 2021;112:3945-52.
https://doi.org/10.1111/cas.15068
Vijay K, Sowmya PR, Arathi BP, Shilpa S, Shwetha HJ, Raju M, et al. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem Toxicol 2018;118:675-90.
https://doi.org/10.1016/j.fct.2018.06.027
Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 2002;30:620-50.
https://doi.org/10.1080/01926230290166724
Yeldu MH, Jibrin A, Ngaski AA, Bashir MB, Mainasara AS, Sani B, et al. Lipid peroxidation and enzymatic antioxidants among breast cancer women of african descent in Sokoto, Nigeria. Ann Res Rev Biol 2017;14:1-8.
https://doi.org/10.9734/ARRB/2017/34672
Pakmanesh F, Moslemi D, Mahjoub S. Pre and post chemotherapy evaluation of breast cancer patients: Biochemical approach of serum selenium and antioxidant enzymes. Caspian J Intern Med 2020; 11:403-9.
Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB. Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med 2015;87:84-97.
https://doi.org/10.1016/j.freeradbiomed.2015.06.017
Walton PA, Brees C, Lismont C, Apanasets O, Fransen M. The peroxisomal import receptor PEX5 functions as a stress sensor, retaining catalase in the cytosol in times of oxidative stress. Biochim Biophys Acta Mol Cell Res 2017;1864:1833-43.
https://doi.org/10.1016/j.bbamcr.2017.07.013
Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014;2014:150845.
https://doi.org/10.1155/2014/150845
Shi Y, Sahu RP, Srivastava SK. Triphala inhibits both in vitro and in vivo xenograft growth of pancreatic tumor cells by inducing apoptosis. BMC Cancer 2008;8:294.
https://doi.org/10.1186/1471-2407-8-294
Vadde R, Radhakrishnan S, Reddivari L, Vanamala JK. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/Cyclin D1 and Elevation of Bax/ Bcl-2 Ratio. Biomed Res Int 2015;2015:649263.
https://doi.org/10.1155/2015/649263
Li Q, Ma Z, Liu Y, Kan X, Wang C, Su B, et al. Low doses of paclitaxel enhance liver metastasis of breast cancer cells in the mouse model. FEBS J 2016;283: 2836-52.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Chulalongkorn Medical Journal

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.









